LFB in the world

Europe
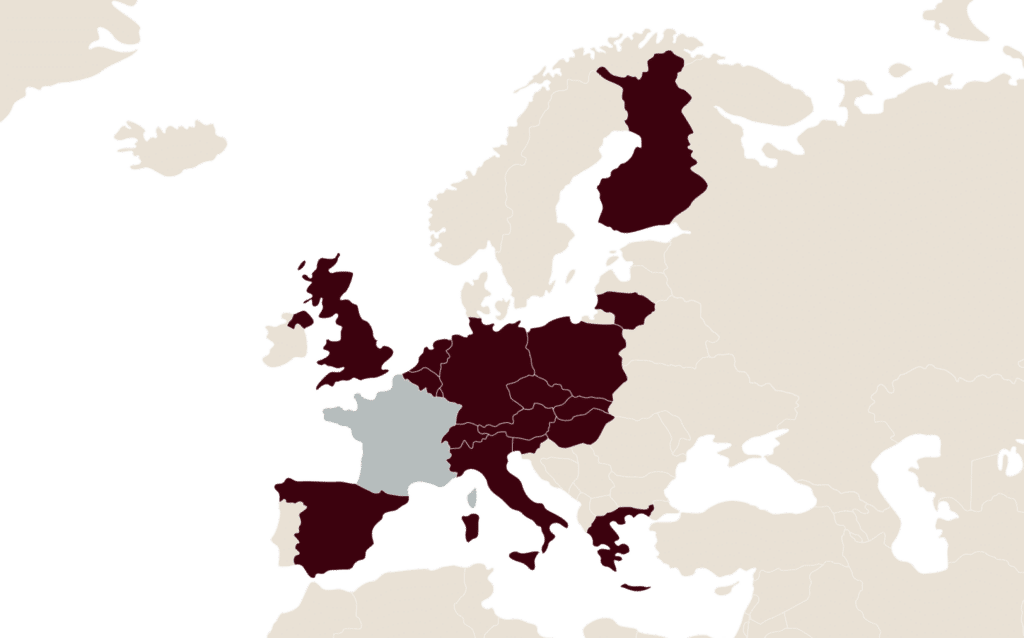
A leader in Europe
LFB has a foothold in various European countries for the marketing of its plasma-derived medicinal products, notably with the presence of subsidiaries in key countries such as Belgium, Germany, Spain and the United Kingdom.
LFB also works to expand its plasma collection network in Europe with 26 plasma and blood collection centres, with the goal of ensuring a continuous supply of plasma to manufacture its medicinal products.
In Europe, LFB has chosen to concentrate its efforts on five countries with growth potential for its key medicinal products.
Today, LFB markets biomedicinal products in around a dozen European countries, with the support of its subsidiaries and commercial partners.
- Austria
- Finland
- Greece
- Hungary
- Lithuania
- Luxembourg
- Netherlands
- Poland
- Slovakia
- Slovenia
- Switzerland
EUROPLASMA, a subsidiary of LFB, manages 26 plasma and blood collection centres in Europe:
- 5 centres in the Czech Republic,
- 9 centres in Austria,
- 2 centres in Germany.
LFB is thus committed to increasing its plasma collection capacity in order to meet the growing demand from patients in Europe. Securing a continuous supply of plasma is a major public health issue.
France
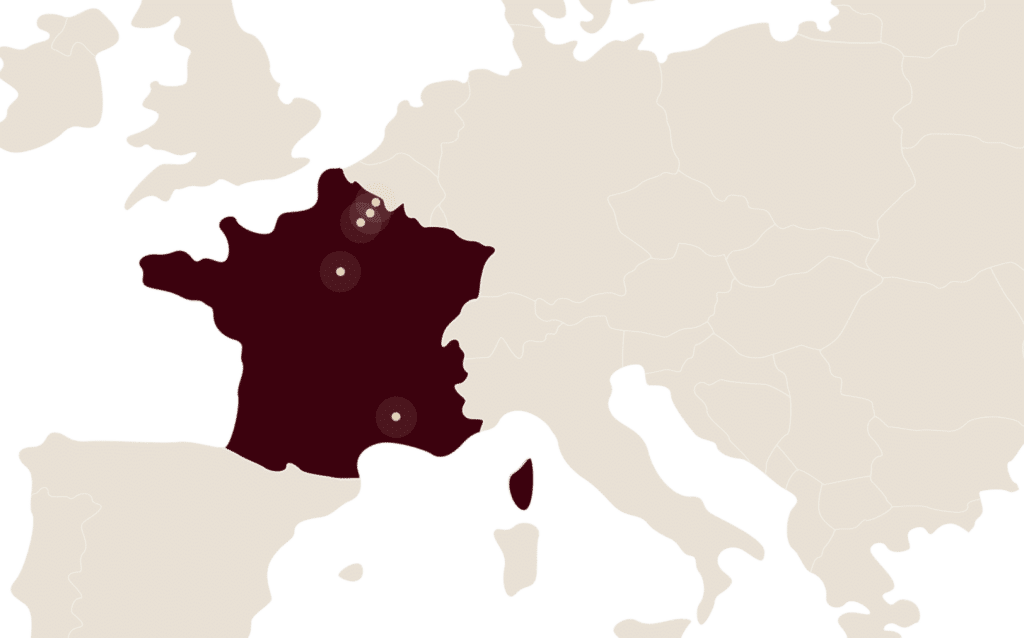
A major economic and industrial player in France
With about fifteen biomedicinal products on the market, LFB is a major player in France for health professionals in hospitals.
LFB’s medicinal products are used to treat hundreds of thousands of patients each year, for both chronic care and in emergency situations.
With 2,100 employees in France, 5 industrial sites, the Group’s head office and development teams, LFB is a major economic player in health.
- Alès
- Arras
- Carvin
- Lille
- Les Ulis
Blood products, including plasma, are collected in France by a single operator: the “Etablissement Français du Sang” (EFS).
In France, donations of plasma, blood and platelets are voluntary, anonymous, unpaid and uncompensated.
Thanks to its CDMO status (Contract Development and Manufacturing Organization), and approved by the FDA (Food and Drug Administration), the Alès site shares its know-how and equipment with its customers, including support from the development of cell lines and industrial-scale processes to the manufacture of clinical and commercial batches, as well as the associated analytical testing. It is one of LFB’s key entities specialising in the bioproduction of therapeutic proteins, in particular monoclonal antibodies, by cell culture.
The Arras site is a new generation plant that houses all of the production steps for 3 medicinal products.
Commissioned at the end of 2024, this plant will enable LFB to triple its production capacity.
With this € 750 million investment, LFB will make a real technological leap forward
The Carvin site (Pas-de-Calais department, France) offers a warehouse to store the materials needed to produce medicinal products for the Lille plant.
It is also the packaging site for LFB’s liquid products.
The Lille plant (North department, France) specialises in the downstream steps of the production processes for plasma-derived medicinal products, from the intermediate products to aseptic filling.
The Les Ulis plant (Essonne department, France) specialises in the upstream steps of the production processes for plasma-derived medicinal products, from receipt of the bags of plasma to the intermediate products.
LFB’s head offices are located in Paris.
Americas
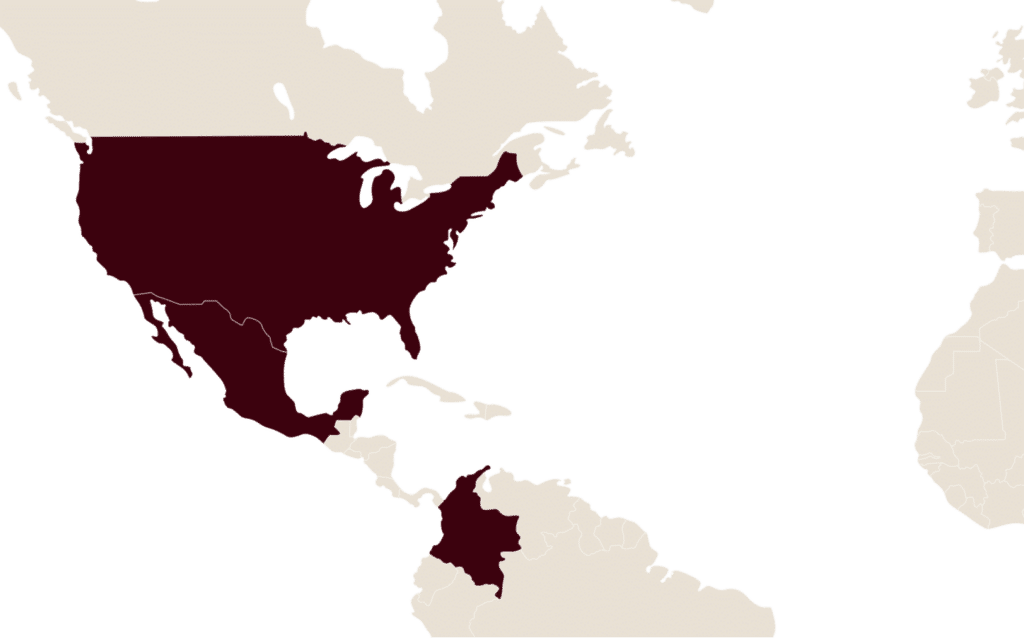
LFB has been established in the Americas for many years, with a bioproduction site, plasma and blood collection centres and products already on the market.
Located in the state of Massachusetts in the United States, the Charlton plant specialises in the production of recombinant proteins developed on its rPRO™ technological platform. This technology consists of expressing the desired protein of interest in mammalian milk by genetic recombination.
LFB and its American subsidiary LFB Plasma manage 6 plasma collection centres in the United States of America: in Alabama, Colorado, Florida, North Carolina and South Carolina.
In accordance with US FDA requirements, “Food and Drug Administration”, only medicinal products derived from plasma donated in the USA can be sold in the USA.
LFB markets a plasma-derived medicinal product in the United States of America.
LFB is also present in Mexico, through its subsidiary LFB MEXICO responsible for the marketing of several plasma-derived medicinal products, and in Colombia.
- Mexico
Other countries worldwide
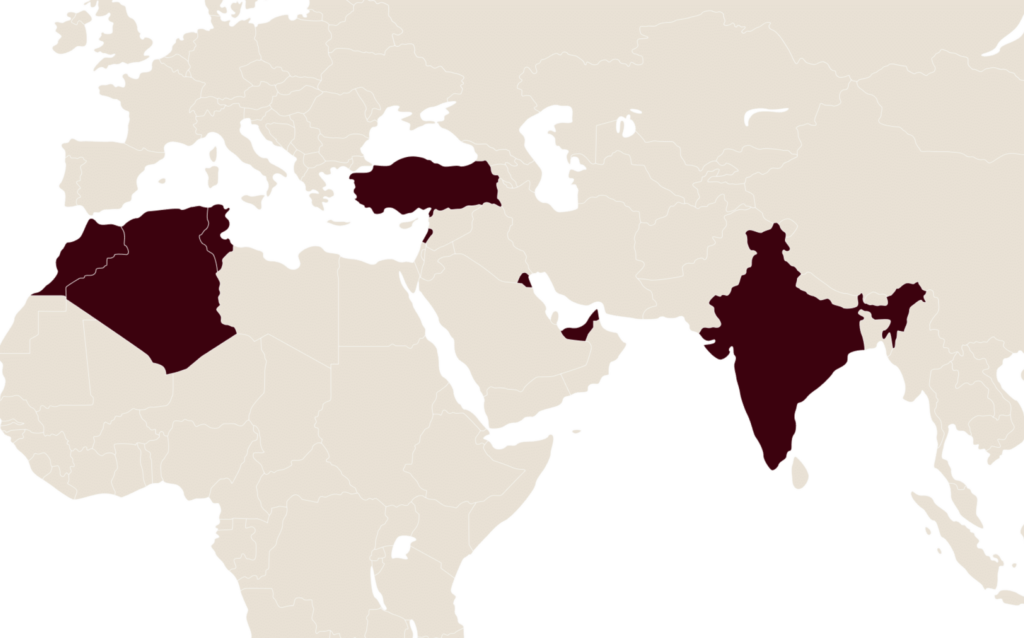
Today, LFB markets biomedicinal products with the support of its subsidiaries and commercial partners.
Turkey is a key growth country selected by LFB for the marketing of its key medicinal products.
LFB also markets its products in some countries in the rest of the world through commercial partners.
- Algeria
- India
- Kuwait
- Lebanon
- Morocco
- Tunisia
- United Arab Emirates
Job Code: NP-25/10/001 – Date of preparation: November 2025




