From Plasma to Biomedicinal Products
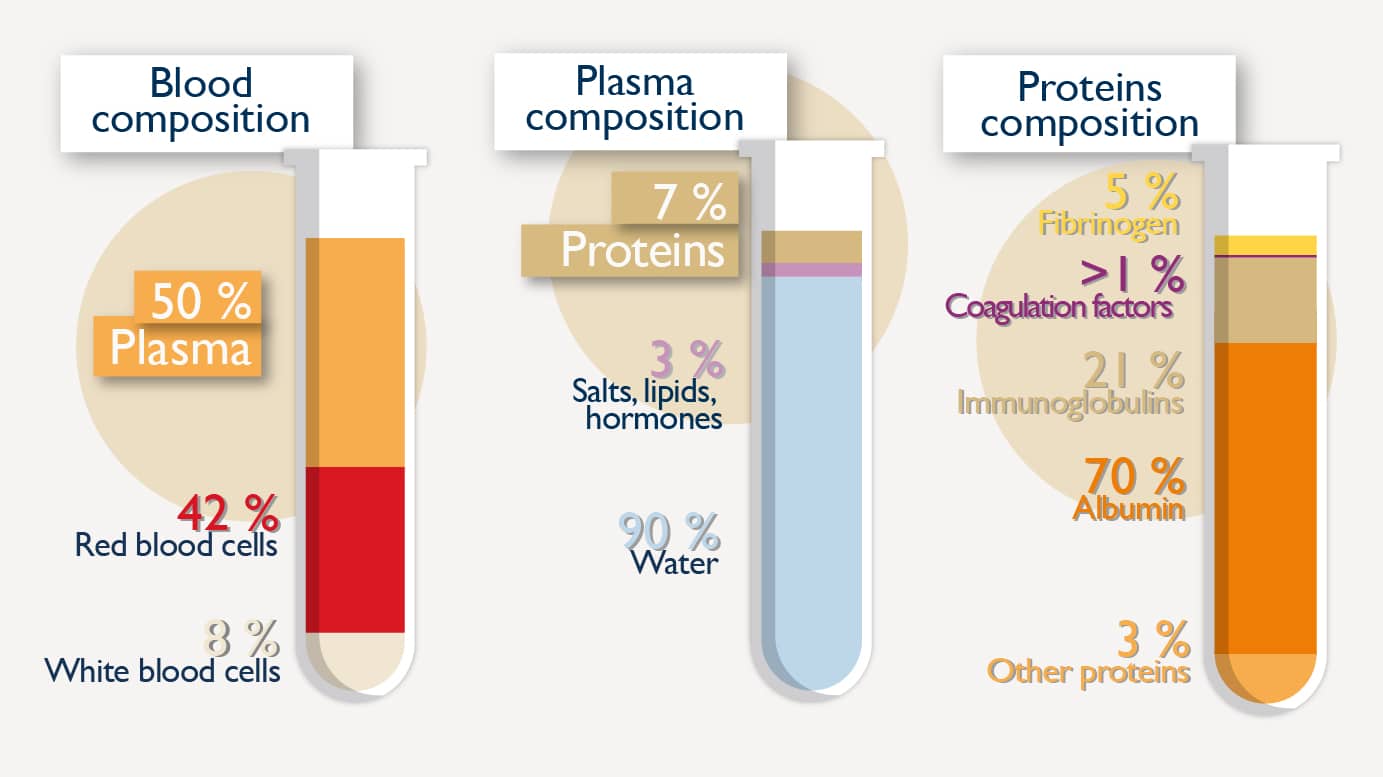
Transforming plasma into medicinal products is called fractionation. Fractionating plasma means purifying, or in other words, separating, isolating and performing safety steps on the therapeutic protein that goes into the final medicinal product.

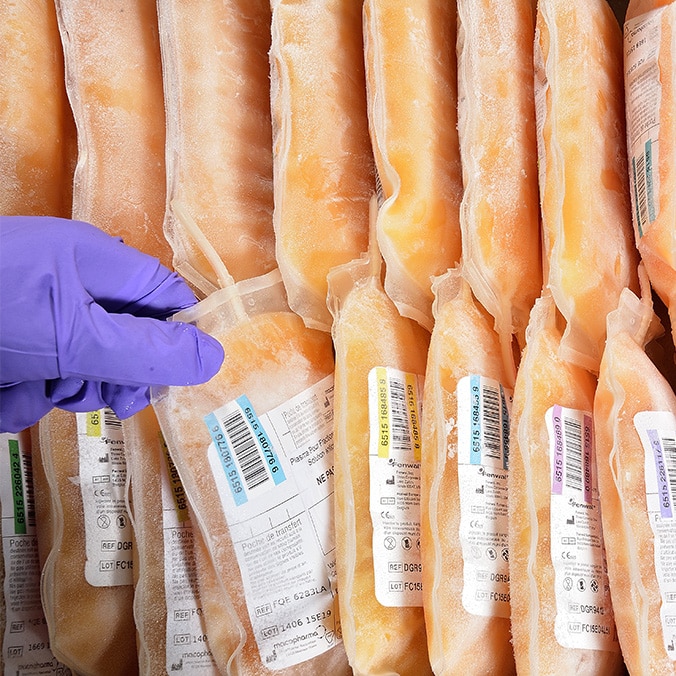
Plasma
Before it arrives at LFB’s plants in bags, the plasma is duly checked and has been certified to meet the highest criteria of quality and biological safety.
For example in France, it is the "Etablissement Français du Sang" that is responsible for collecting and qualifying donations and preparing labile blood products.
Fractionation
LFB’s medicinal products are produced using a manufacturing process called fractionation, which involves isolating, purifying and helping ensure the safety of proteins, some of which are present in very small quantities in human plasma.
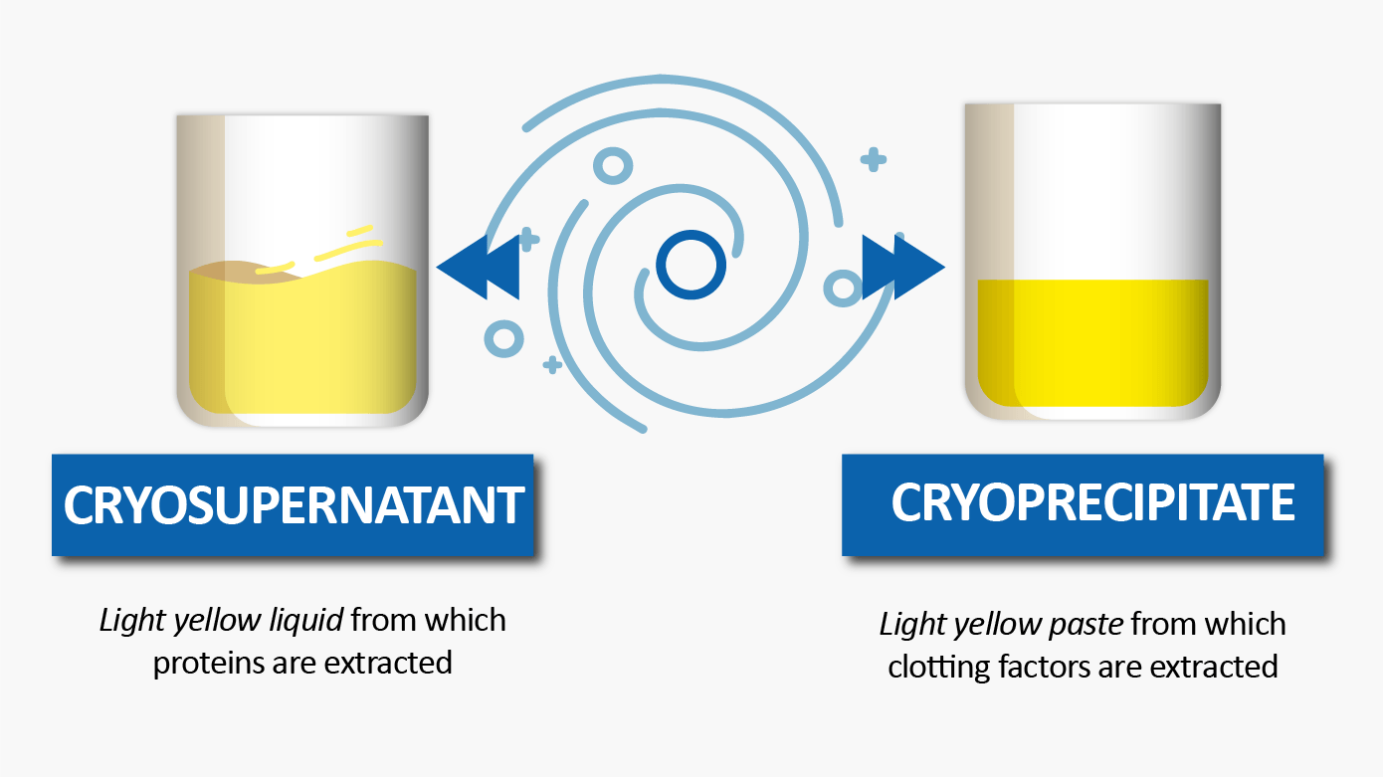
Fractionation begins with Cryoseparation:
Several specific production techniques are used to extract the desired protein from plasma:
For example, from the cryosupernatant, ethanol precipitation, filtration and then ultrafiltration are performed to isolate and concentrate intermediate products.
For each protein of interest, a dedicated chromatography is implemented.
Biological safety steps for medicinal products
During its production processes, LFB implements biological safety steps that act specifically or contribute to the inactivation and elimination of infectious agents, as certified by pharmaceutical regulations.
Purification and isolation of the protein of interest
Specific viral inactivation step by physical or chemical treatment:
- Dry heating
- Pasteurisation
- pH 4 / Pepsin
- Solvent / detergent treatment
Specific step to remove infectious agents: nanofiltration
Manufacturing step that contributes to the elimination and/or inactivation of infectious agents:
- Ethanol or caprylic acid precipitation,
- Chromatographies,
- Absorption on an alumina gel, precipitation, filtration,
- Depth filtration.
Taken together, this system helps guarantee the efficacy and tolerability of medicinal products.
Pharmaceutical formulation: the final step in production.
After a final sterilising filtration, the product is filled into vials.
All of the medicinal products are then inspected visually by technicians trained specifically to detect any possible contamination and/or deterioration of the product.
Most of LFB’s medicinal products are then freeze dried, meaning that the liquid is “removed” to make the medicinal product solid, for better storage and stability.
Job Code: NP-25/10/001 – Date of preparation: November 2025